MENU
The KH SARS-COV-2 Detection Kit is an in vitro diagnostic medical device, based on
Real-time RT-PCR technology utilizing reverse-transcriptase (RT) reaction to convert RNA into complementary DNA (cDNA).
| KH SARS-COV-2 Detection Kit (REF: RV010) | |
|---|---|
| Intended use | Qualitative detection of RNA (S gene and RdRp gene) of SARS-CoV-2 from nasopharyngeal and oropharyngeal swab specimens to aid in the diagnosis of SARS-CoV-2 infection |
| Specimen type | Respiratory specimens such as nasopharyngeal or oropharyngeal swabs specimens from symptomatic individuals suspected of COVID-19. |
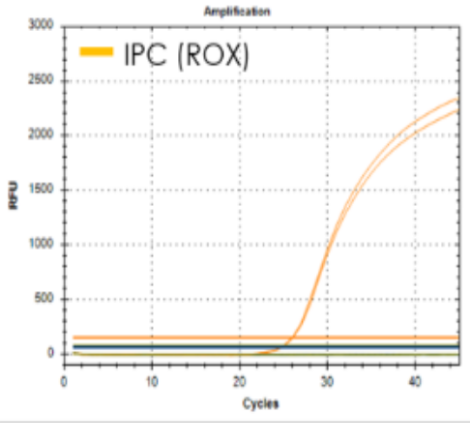
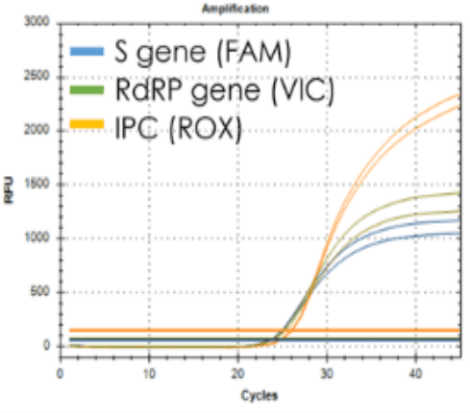
| Target | S gene and RdRp gene |
| Product contents |
SARS-CoV-2 Primer & Probe Mixture 3X RT MasterMix SARS-CoV-2 Positive Control RNase free Water SARS-CoV-2 Extraction Control* |
| LoD (copies/㎕) | 0.66 copies |
| Storage condition |
-25 ℃ ~ -15 ℃ |


* Simplified Procedure when make mixture
(Number of tubes minimized)!